Hydrogen Production by
Rhodobacter Sphaeroides O.U.001
Introduction (TOP)
Hydrogen is a clean and efficient fuel, and a potential
substitute for fossil fuels in the long run.
Biological hydrogen production is environmentally friendly, uses
renewable resources and does not require complex equipment
Currently low production rates and high substrate costs limit the
economical feasibility of biological hydrogen production. Increasing
feasibility requires detailed research.
Objectives (TOP)
Studies made by our group aim the following:
Development of an understanding of the hydrogen production
characteristics of the bacteria
Increasing the hydrogen production rates through optimization of
variables
Investigation of the possibilities of decreasing the cost
Scaling-up of the photobioreactor for the biological hydrogen
production.
Microorganisms Producing
H2 (TOP)
Algae (Oxygenic photosynthesis)
H2O
à H2
+
½ O2
Cyanobacteria (Oxygenic photosynthesis)
H2O
à H2
+
½ O2
Anaerobic bacteria (Fermentation)
C6H12O6
à H2+
CO2+
organic
acids
Photosynthetic bacteria (Anoxygenic
photosynthesis)
organic
acids
+
H2O
à H2
+
CO2
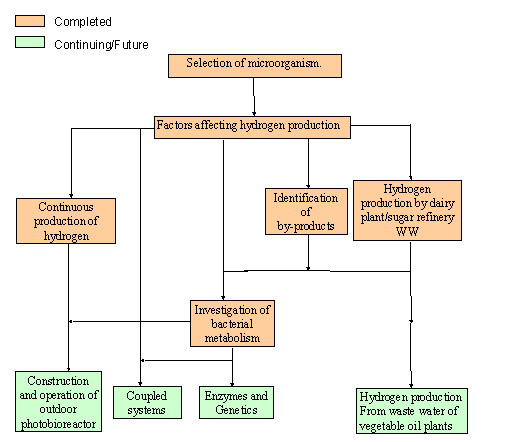
Studies
of the M.E.T.U. Biohydrogen group with R.sphaeroides OU 001(TOP)
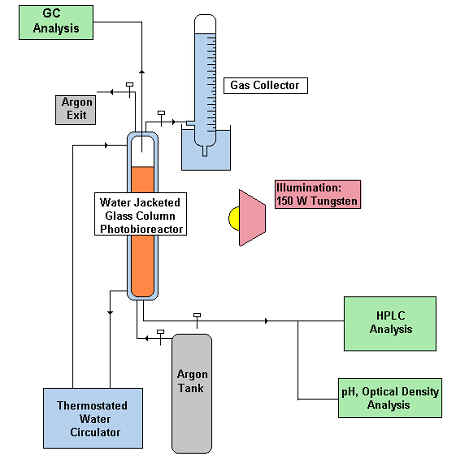
Hydrogen
Production Setup (TOP)
Typical Conditions
(TOP)
The photosynthetic bacteria evolve hydrogen from
organic carbon compounds when subjected to illumination under an
inert anaerobic atmosphere. Below are typical conditions used in
hydrogen production experiments.
Temperature: 30-35 °C
Initial pH: 6.8-7.0
Nutrient medium: (Modified from Biebl and Pfennig,
1981)
Malate / Wastewater (Carbon) Glutamate (Nitrogen)
Salts+Vitamins
Light Intensity: (Tungsten)150-250 W/m2
Atmosphere: Pure argon
Volume: 400 ml
Selection of
microorganism (TOP)
Each group of microorganism has its advantages and
drawbacks for hydrogen production. The following advantages of
Photosynthetic Bacteria make them particularly suitable for
biological hydrogen production.
High substrate conversion efficiency.
Ability to utilise a wide wavelength range of
sunlight.
Ability to utilise a large variety of compounds
for growth or H2 production.
Ability to survive under changing conditions. Among
the photosyntetic bacteria, R. sphaeroides was observed to have a
high hydrogen production rate. Therefore this bacterium was selected
and studied for hydrogen production.
Factors
Affecting Hydrogen Production (TOP)
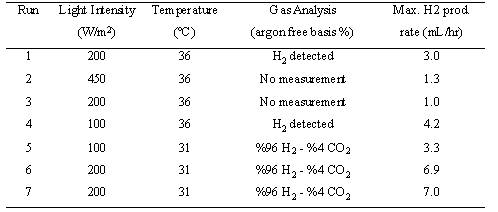
Effect of temperature and light intensity
There are many factors which influence hydrogen (Arik et.
al.,1996) production by R. sphaeroides in one way or the other.
First, the effect of parameters that could be easily manipulated and
set constant- such as temperature, light intensity- were
investigated.
Table 1. Influence of
temperature and light intensity
Effect of Substrate Concentrations
Then the effect of substrate concentrations was
examined (Eroğlu et. al.,1999). It was found that high
concentrations of glutamate promoted growth at the expense of
hydrogen production. The effect of glutamate concentration is seen
in Figure 2.
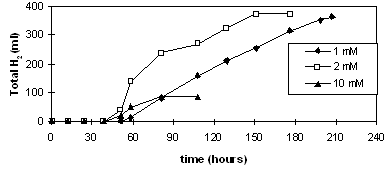
Figure 2 Hydrogen
produced with 15 mM malate and 3 different glutamate concentrations.
Also in this work, kinetic relations between substrate
consumption and hydrogen utilisation were proposed.
Use of Yeast Extract
Use of yeast extract to replace the standard vitamin solution
resulted in an increased rate of hydrogen production (Figure 3)
Moreover, the production started earlier.
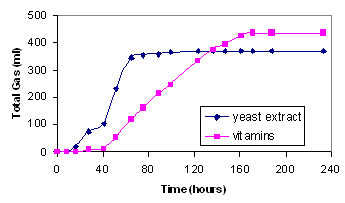
Figure 3 Comparison of
hydrogen production with yeast extract (0.2 gl) with that of the
standard vitamin solution
Use of light-dark cycles
Growth and hydrogen production were minimal in the
dark periods but the use of alternating light-dark periods resulted
in an increase in hydrogen produced compared to continuous
illumination.
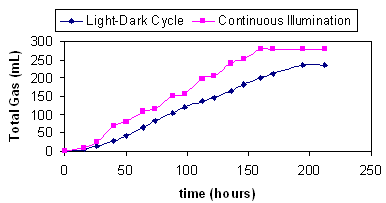
Figure 4 Comparison of
hydrogen production in photobioreactor exposed to light-dark cycles
(14h-10h) with hydrogen produced in a continuously illuminated
photobioreactor
Continuous
Hydrogen Production (TOP)
By the addition of bacteria and medium with regular
intervals, continuous hydrogen production for long periods, e.g. for
more than two months, was accomplished. Figure 5 shows one of these
runs. (Eroğlu et. al., 1998)
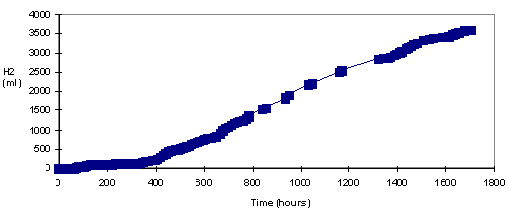
Figure 5 Continuous
hydrogen production by diluting the reactor with 100 ml of fresh
medium and 30 ml bacterial culture every 100 hours.
Use of Wastewater
(TOP)
The cost of the biological hydrogen production can
be decreased by supplying at least a part of the nutrient
requirements for the bacteria by wastewater. Hydrogen production
experiments with the waste water of two common industries-sugar
refinery and dairy plant- were conducted.
Use of dairy plant waste water (Türkarslan et. al., 1998)
:
Waste water on its own did not support bacterial growth. A blend
of waste water with the standard medium resulted in increased growth
and increased hydrogen production compared to the standard medium.
Use of sugar refinery waste water (Yetiş et. al.,
2000):
Hydrogen production was observed in blends of waste water with
the standard medium. Highest hydrogen production rate was obtained
in 20 % waste water. (Figure 6)
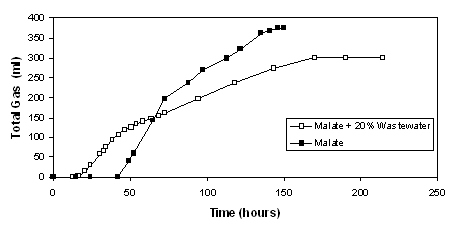
Figure 6 Hydrogen
produced by R. sphaeroides using malate only and a blend of malate
and 20 % sugar refinery wastewater.
By-products
(TOP)
Identification and isolation of useful products
other than hydrogen may increase overall feasibility and provide
valuable information on metabolism. Poly-beta-hydroxybutyric acid (PHB),
a biodegradable polymer, is one of the by-products of Rhodobacter
sphaeroides O.U. 001. PHB can be used for production of disposable
bags and containers. Biocompatibility of PHB makes it a desirable
material for medical applications such as controlled drug release
and the production of surgical pins, wound dressing, blood vessel
replacements etc.In R. sphaeroides, PHB is accumulated as an
intracellular carbon and energy storage material. PHB is accumulated
as granules localized at different sites of cytoplasm. An electron
micrograph of PHB granules in R. sphaeroides is seen at the left
(Figure 7).
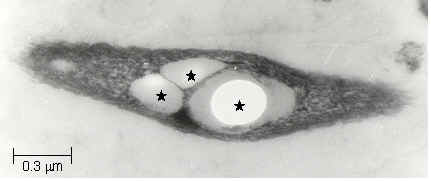
Figure 7. Electron
micrograph of PHB granules (*) in Rhodobacter sphaeroides O.U. 001,
fixed with Glutaraldehyde and Uranyl acetate, dehydrated with
Acetone, stained with Lead citrate.
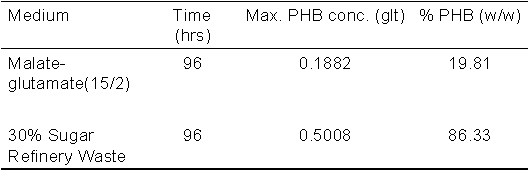
An example PHB production curve of R. sphaeroides
O.U.001 as a function of time is given in Figure 8. R. sphaeroides
was found to produce more PHB in a blend of sugar refinery waste
water and malate, than from malate alone. This comparison is
displayed in Table 2.
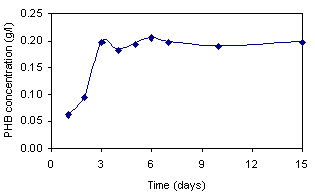
Figure 8. Production of PHB
as a function of time by Rhodobacter sphaeroides O.U. 001 in malate/
glutamate medium
Table 2: Accumulation of PHB
in various media
Metabolism
(TOP)
Information on metabolism is essential to understand
the mechanisms involved in hydrogen production and to identify the
limiting factors. The following information has been obtained from
the literature and from conducted experiments. Hydrogen production
occurs through the operation of a light-dependent, anaerobic TCA
cycle. Electrons produced from the substrate by the TCA cycle are
transferred by electron carriers to the nitrogenase. The nitrogenase
uses these electrons to reduce protons.
Flow of electrons:
Substrate à(TCA
cycle) à
NADàFerredoxinàNitrogenase enzyme
Nitrogenase Reaction: 2H+ + 2e- + 4ATPà H2
R. sphaeroides is capable of several alternative
pathways such as aerobic respiration and phototoautotrophy. Hydrogen
production is practically absent for these pathways. R. sphaeroides
can use a very large variety of carbon and nitrogen sources such as
sugars, organic acids, glycerol etc. for growth. However, substrates
differ greatly in their manner of utilisation, therefore substrates
which promote the hydrogen production pathways are very limited.
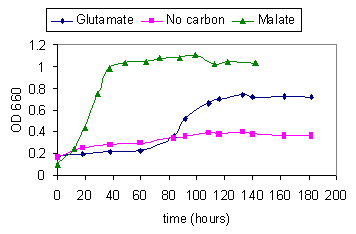
Figure 9. Growth curves of R.
sphaeroides O.U.001 with: i)Malate as the carbon source(15 mM) and
glutamate as the nitrogen source(2 mM), ii) Glutamate (2mM) as the
sole carbon and nitrogen source and iii) No carbon source, only
ammonium chloride (2mM) supplied.
Figure 9 illustrates the growth of R. sphaeroides
under various conditions of carbon availability. Malate+glutamate is
the preferred medium. When glutamate is the only carbon and nitrogen
source, cells still grow appreciably. When only ammonium chloride is
present (no carbon source given) the bacteria still grow to some
extent, possibly by utilising their endogeneous reserves, such as
PHB.
Conclusions
(TOP)
High purity (95-99%) hydrogen has been produced in
reactors designed specifically for the purpose.
Variables such as temperature, light intensity and
substrate concentration have been optimized for hydrogen
production.
Kinetic expressions relating substrate consumption
and hydrogen production with substrate concentrations have been
proposed.
Experiments have been conducted in which long term
continuous hydrogen production has been accomplished.
Hydrogen production was found to be possible in
specific blends of wastewater with the standard medium.
The valuable by-product poly-hydroxybutyrate has
been detected and identified.
Future Plans
(TOP)
Improvement of hydrogen production through
optimisation of metabolism. For this purpose investigation and
identification of the genetic structure is also necessary.
Construction and continuous operation of an 8 liter
outdoor photobioreactor.
Investigation of the effect of other parameters,
such as the use of coupled R. sphaeroides-Bacteriorhodopsin systems.
Completion of the characterization and
identification of PHB.
Investigation of the possibility of hydrogen
production from vegetable oil waste.
References
(TOP)
Arık, T., Gündüz, U., Yücel, M., Türker,
L., Sediroğlu, V., Eroğlu, İ., "Photoproduction
of hydrogen by Rhodobacter sphaeroides O.U.001", Proceedings of
the 11th World Hydrogen Energy Conference, Stuttgart, Germany, Vol
3, 2417-2424, 1996
Biebl, H., Pfennig N., "Isolation of Members of
the Family Rhodosprillaceae" M.P. Starr, H. Stolp, H.G. Trüper,
A. Balows, H.G. Schlegel (eds.) The Prokaryotes, Springer-Verlag,
New York, Vol. 1, 267-273, 1981
Eroğlu, İ., Aslan, K., Gündüz,U., Yücel,
M., Türker, L., "Substrate consumption rates for hydrogen
production by Rhodobacter sphaeroides in a column photobioreactor",
J. Biotech., 70: 103-113, 1999
Eroğlu, İ., Aslan, K., Gündüz, U., Yücel,
M., Türker, L., "Continuous hydrogen production by Rhodobacter
sphaeroides O.U.001.", O.R. Zaborsky (ed.) Biohydrogen, Plenum
Press, New York, 143-150, 1998.
Türkarslan, S., Yiğit, DÖ., Aslan, K., Eroğlu,
İ., Gündüz, U., "Photobiological hydrogen production by
Rhodobacter sphaeroides O.U.001 by utilization of waste water from
milk industry.", O.R. Zaborsky (ed.) Biohydrogen, Plenum Press,
New York, 151-156, 1998
Yetiş, M., Gündüz, U., Eroğlu İ., Yücel,
M., Türker, L., "Photoproduction of hydrogen from sugar
refinery wastewater by Rhodobacter sphaeroides O.U.001", Int.
J. Hyd. Eng., 25: 1035-1041, 2000
Yiğit, D.Ö., Gündüz, U., Türker, L., Yücel,
M., Eroğlu, İ. "Identification of by-products in
hydrogen producing bacteria; Rhodobacter sphaeroides O.U.001 grown
in the waste water of a sugar refinery." J. Biotech.
70:125-131, 1999.
[ Home ] [ Members ] [ Researches ] [ Publications ] [ Photos ] [ E-mail ]
|
